How a woman-led biotech company is advancing medical cannabis
Millions of women around the world live with an incurable and mistreated condition, and many turn to cannabis products for relaxation – despite the disproportionate effects and risks of unlabeled products.
For Melissa Sturgess, CEO of biotech-based Ananda Pharma, this situation presents an opportunity, she told Mjbizdaily.
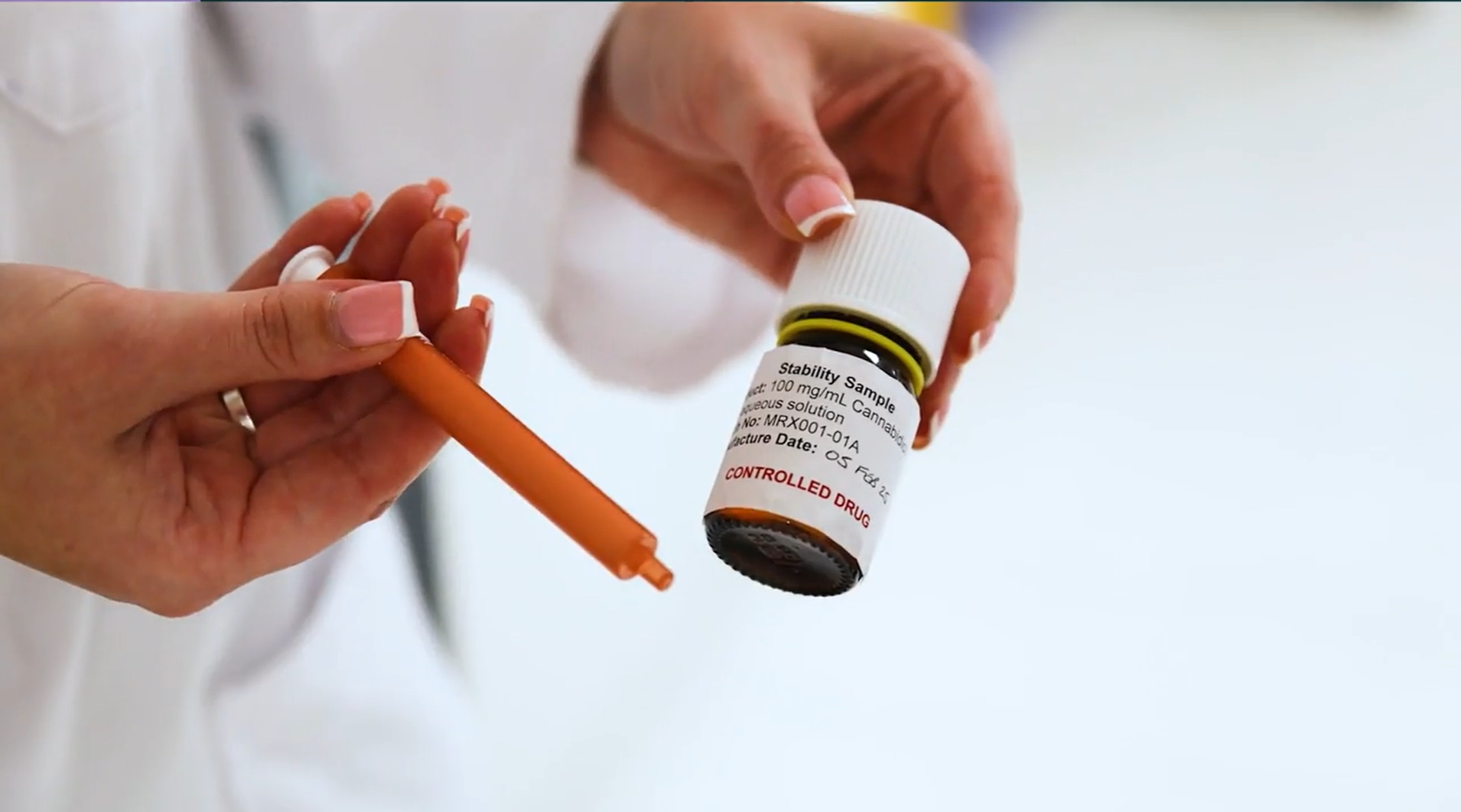
Ananda Pharma is using CANARMACEUTICAL-grade cannabinoid research to develop what could be the first CBD treatment approved by the Regulator for Endometriosis, a painful condition in which uterine tissue grows outside the uterus.
This approach reflects a wider trend in cannabis: credibility depends on evidence, not promises, a point that is becoming difficult for the industry to ignore.
That’s a layer of content it will expand on next month at mjbizcon in Las Vegas.
Along with other women leading health-focused companies in the cannabis space, she will explain why the future can be made for those who work by finding their impact and returning all claims for results.
A painful condition, without a cure – treated with cannabis
Endometriosis affects about 10% of women of reproductive age worldwide, according to the World Health Organization. This chronic disease has no cure and treatment is often limited to symptom management.
It is also very researched. Only 0.038% of the national institutes of health research budget is dedicated to research on the condition, according to the latest estimate.
To fill the gap, women are turning to CBD products for help.
But there are only certain classes of drugs, CBD-based medicine. And currently, epidiolex can only be prescribed to treat severe epilepsy.
Meanwhile, most hemp-oil formulations available are sold over the counter in the United States, including at smoke shops and gas stations. That means unthinking buyers don’t always know what they’re getting.
And the situation is not much better even within the operations of the state-controlled cannabis markets.
Women have been searching for cannabis-based medicine
Since cannabis remains illegal, research is limited, and there will be cannabis medicines blocked from the expensive food and drug approval process (FDA).
Cannabis products that make any claims to treat a condition or provide health benefits run the risk of fines and penalties.
Despite all of this, women kept coming to their doctors and asking for CBD, Sturgess said.
And so the doctors went to Sturgess.
“We were approached by specialists who were asked by patients to prescribe CBD for their chemotherapy-induced neuropathy and endometriosis,” she said.
“They came to us looking for a formulation that could be used in clinical trials.”
For Sturgess, that was a turning point.
He realized broad patient access to medical cannabis would only come through systematic regulation.
Ananda Pharma pursues Cannabis-based research
With its CBD-based treatment, Ananda is pursuing positive approaches to drug discovery in the UK, where most healthcare flows through the National Health Service (NHS).
Unlike the US, where full health insurance coverage for medical marijuana remains a distant dream, the NHS covers medical marijuana.
However, the NHS covers cannabis for a very small number of people – and only for a small number of conditions.
Endometriosis is not one of them.
That’s mainly because the NHS only reimburses for drugs that have been proven in randomized, controlled trials.
With medicinal cannabis, the motivation is clear: Prove efficacy, or remain without routine care.
The success story with epidiolex shows drugs derived from naturally-derived cannabinoids can meet the full regulatory standards while delivering value to patients and investors, Sturgess said.
Building on that premise, Sturgess focused Ananda’s efforts on one issue: the pain associated with endometriosis.
The ultimate goal is to develop their lead formulation, MRX-1, into a clinically available and reversible treatment.
The way forward is medicinal cannabis
To do that, Ananda follows a typical biotech model with placebo-controlled designs, standardized designs, and measurement methods.
Ananda reached its first milestone this year with a healthy volunteer course where 20 participants were organized under the supervision of the hospital.
Findings from early clinical trials will inform a larger patient trial planned for 2026.
If successful, MRX-1 could be the first cannabinoid-based drug developed for women’s health pain.
Sturgess says the milestone is already meeting with investors as regulators in the US and across the country move toward clear medical areas for cannabinoids.
Subscribe to MJBIZ TRCROCK
Exclusive industry data and analysis to help you make informed business decisions and prevent costly missteps. All facts, no hype.
What you will get:
- Monthly and quarterly updates, with new data and insights
- Financial Forecasts + Financial Investment Trends
- A state-by-state guide to the state of regulations, taxation and market opportunities
- Annual survey of cannabis businesses
- Consumer understanding
- And more!
Lessons from biotech in the cannabis sector at Mjbizcon
The company’s initial development drew attention, as Sturgess was spotted attending a women’s and lifestyle-focused conference in June.
“Everybody said, ‘FinallyTo do some work,'” He remembers.
Ananda’s approach reflects a broader approach to cannabis. Convincing is about asking, not hype or promises.
At MJBIZC – where Sturgess and other three startups from health-focused companies will speak at the Scoent Symposium on Tuesday, Dec. 2 – Testimonials may have fallen to the floor of the showroom.
Strengthening margins and the rise of investors’ consideration forces those who work by force – from agricultural technology to consumer goods – to return to the back of measurable data.
Sturgess’ advice for providers preparing to compete in a data-driven future is simple.
“Stay focused,” she said. “There are a lot of shiny distractions in this space, but stay true to your mission and overall data.”




